Seeing is Believing
EMBL Heidelberg was the venue for the EMBL Symposium: Seeing is Believing – Imaging the Molecular Processes of Life that took place on 9-12 October 2019. This was the second EMBL meeting on imaging data we attending this year after VIZBI (see the write-up here), GigaScience Data Scientist Chris Armit was there and was astonished at how recent breakthroughs in imaging technology are enlightening our understanding of the Life Sciences. Here is his GigaBlog take on the highlights of the symposium.
The Devil is in the Detail: Super-Resolution Imaging and Electron Microscopy
A major focus of this symposium was super-image resolution, the huge datasets coming from this a particular area of interest for GigaScience. With us publishing Data Notes showcasing this data and recently attending the Super Resolution Clinic in Edinburgh. Conventional resolution of a light microscope is diffraction limited to approximately 200 nanometre in the lateral dimension and approximately 500 nanometre in the axial dimension. What this means is that the diffraction limit of a microscope hinders the ability to see single molecules as the optics do not allow the researcher to distinguish between two fluorescently labelled molecules that are less than 200 nanometre apart. Super-resolution microscopy allows researchers to go beyond the diffraction limit and image sub-cellular nanostructure. With two Nobel Laureates – Stefan Hell and William Moerner – attending this EMBL Symposium, there was immense expertise at this event.

Cells stained for tubulin (cyan) and actin (magenta) imaged with a novel scalable super-resolution method. Zoom-in and super-resolved image reconstruction. Images by Ola Sabet (2019) and reproduced with kind permission of Ola Sabet and the Pelkmans lab.
Stefan Hell of the Max Planck Institute for Biological Chemistry – who together with Eric Betzig and William Moerner received the Nobel Prize in Chemistry in 2014 “for the development of super-resolved fluorescence microscopy” – provided an exploration of a new powerful super-resolution concept named MINIFLUX microscopy that offers jaw-dropping molecular resolution images of fluorescently labelled probes in living cells. MINIFLUX utilises a local excitation intensity minimum, in the form of a torus or a standing wave, to generate images with 1-3 nanometer resolution, which is the resolution needed to visualise a single molecule.
William Moerner of Stanford University reported on recent advances in 3D super-resolution and Correlative Cryo Light and Electron Microscopy (cryoCLEM) and how these have been used to explore protein superstructures in the primary cilium – an organelle present in most eukaryotic cells that is now thought to have a sensory function – and additionally protein superstructures in bacteria. These showcase examples are thoroughly enlightening, and illuminate our understanding of subcellular structure and the relationship between organelles and macromolecular complexes.
Correlative Light and Electron Microscopy (CLEM) was a major theme of this symposium. Why this is so important is because, with super-resolution imaging, there is still the issue of interpreting what is being looked at. Harald Hess of HHMI Janelia Research Campus – who with Eric Betzig famously developed the first prototype Photo-Activated Localization Microscope (PALM) in Harald’s front room – stated the problem as fluorescence microscopy generating images of “glowing blobs in dark space”. What is needed to correctly interpret these images is a spatial biological framework for these glowing blobs. This is where scanning electron microscopy (SEM) provides the much-needed subcellular context. Focused ion beam SEM (FIB-SEM) and serial block-face SEM (SBEM) are two different electron microscopy techniques that are used to deliver ultrastructural images where morphological subcellular structures can be delineated. SEM typically generates images with 2-6 nanometre lateral resolution. The concept of CLEM is thus very straightforward and it is quite simply to align the morphological structures, as observed by electron microscopy, with fluorescently labelled structures. As Lucy Collinson of The Francis Crick Institute eloquently explained, “Correlative Light and Electron Microscopy (CLEM) combines the benefits of fluorescence and electron imaging, allowing researchers to track rare biological events in the context of cell structure.”
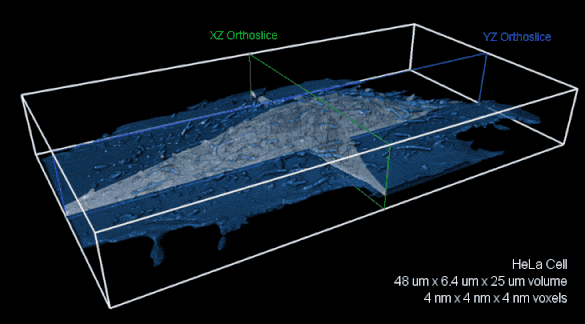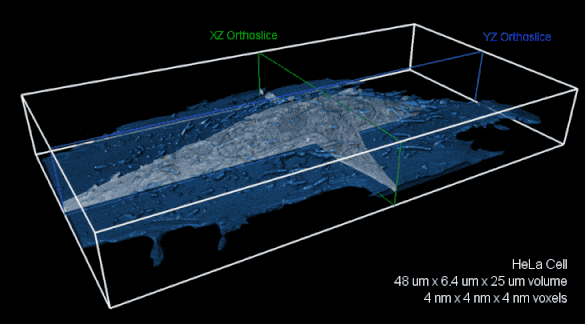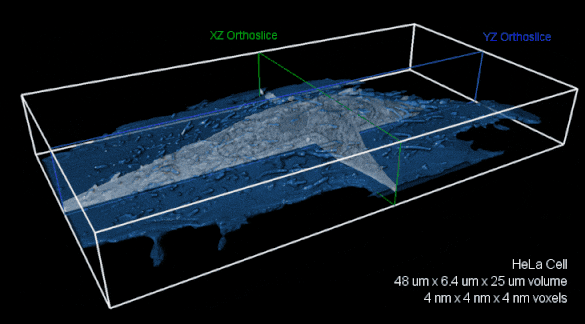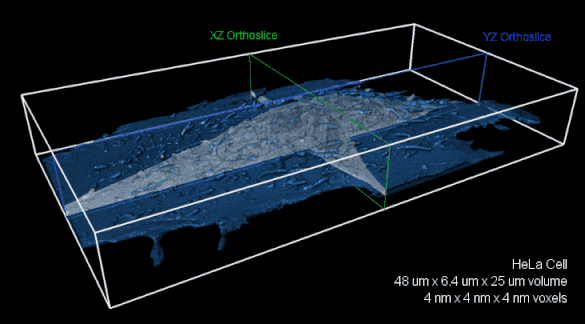
Whole-cell FIB-SEM volume with 4 nm isotropic voxels. XZ (green) and YZ (blue) orthoslices of raw FIBSEM data shown with the cell membrane overlaid in transparent blue.The entire volume is nearly 8,000 cubic microns. Image reproduced with kind permission of the Hess Lab.
Harald Hess presented on the use of CLEM in vitreously frozen COS-7 cells, and using a combination of cryo-SMLM (Single-Molecule Localization Microscopy) and FIB-SEM, Harald showcased breathtaking examples of 3D subcellular compartmentalization where one could observe such filigree detail as the endoplasmic reticulum lumen and the mitochondrial outer membrane in correlated images. Harald explained the utility of FIB-SEM “where fine sequence of 4-8 nanometre increments are ablated off a sample surface and each such surface is imaged with the SEM”. However, the development of FIB-SEM was not without its problems. As Harald explained, in its original manifestation, FIB-SEM was not reliable for more than a few days, and there was major work required behind-the-scenes to tame the focused ion beam and mitigate damage. In addition, FIB-SEM cannot mill more than 50 microns, which is not an issue for imaging cultured cells, but is an issue for imaging tissue. The solution here is to section tissue at 50 micron thickness prior to scanning.
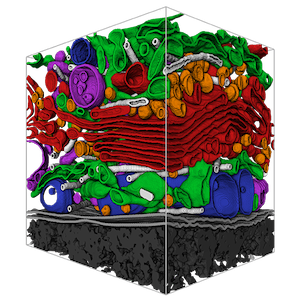
Volume rendered manual segmentations of a cubic micron FIB-SEM volume showcasing organelles. Image by COSEM, and reproduced with kind permission of Aubrey Wiegel.
Aubrey Weigel, a colleague of Harald’s at of HHMI Janelia Research Campus, provided an incredible overview of the COSEM project (Cellular Organelle Segmentation in Electron Microscopy), which aims to delineate organelles in 3D FIB-SEM datasets. As Aubrey explained, “a purely manual segmentation is too-time consuming for large volumes” and therefore the COSEM team “are developing tools for automated identification of all intracellular structures within isotropic FIB-SEM data.”
I caught up with Harald Hess and Aubrey Weigel at the EMBL “Meet the Speakers” session. Harald and colleagues at HHMI Janelia Research Campus are using FIB-SEM to deliver a connectomic circuit model of the Drosophila brain. This is a project of dizzying complexity. The fly brain has a volume of 40 million cubic microns (see the video from HHMI), and the intention here is to capture the connections at 8 nanometre isotropic voxel resolution. Volume rendering will enable connection circuits to be modelled, and these wires and circuits will be further subdivided into excitatory and inhibitory connections. As Harald explained, “FIBSEM is not at the resolution of TEM (Transmission Electron Microscopy), but can look at larger samples.” The project began as a study of the fly hemi-brain, but is now moving towards the entire Drosophila brain. In collaboration with Google, deep learning classifiers are being used to segment and categorise the dendritic connections, and these connectomic maps then need to be validated by experts to ensure that they are accurate.
Aubrey explained to me how the COSEM team segment organelles in a biologically meaningful way using appropriate classifications. “The ER (endoplasmic reticulum) has to link back to itself, otherwise it will be categorised as an endosomal network, or a peroxisome.” In addition, Aubrey pointed out that morphology alone cannot distinguish between early endosomes and late endosomes, and that there is still a need for probes and markers to classify the maturation of membrane-bound endocytic organelles. Aubrey further highlighted how the COSEM atlases – such as the HeLa cell atlas, the macrophage cell atlas, and Jurkat cell atlas (Jurkat cells are an immortalized T-cell line) – will allow us to capture accurate counts of the number of each and every organelle in these different cell types, and so we will have accurate measures of, for example, membrane-bound ribosomes versus cytosolic ribsosomes. In addition, the COSEM delineation effort will allow us to visualise interactomic relationships, such as the sites of contact between ER and mitochondria. Active mitochondria are speculated to have denser cristate, and in the segmented atlas models it will be additionally possible to assess whether various interactomic relationships impact on the density of mitochondrial cristae.
Observing through Time: Live Imaging
At the EMBL Seeing is Believing Symposium, there was great interest in live imaging. In live imaging, the focus is on temporal resolution at the expense of spatial resolution, and this allows researchers to directly observe biological processes over time, which is incredibly informative. Atsushi Miyawaki of the RIKEN Center for Brain Science astonished the audience with his ability to visualise synchronous neural firing in mammalian olivocerebellar segments. This region of the brain is responsible for cerebellar motor learning and function. Atsushi’s team have proven ability in creating biologically relevant sensors, such as the FUCCI (Fluorescent ubiquitination-based cell cycle indicator) system that provides a ‘traffic light’ red-to-green signal change to indicate the cell cycle transition from G1 to S/G2/M in mammalian cells and tissues. In the new in vivo imaging system that was showcased, Atsushi’s team have re-engineered the firefly luciferase system – which utilises the substrate luciferin to generate photons – by modifying both the luciferase gene (the modified gene is called AkaLuc), and by creating a more permeable alternative substrate for luciferase called AkaLumin. This new system, called AkaBLI, has been reported to generate a “bioluminescence signal 1000 times stronger than that from the natural luciferase-luciferin reaction”.
Live imaging can additionally help us understand the mechanisms of disease. Ronald Germain of the US National Institute of Allergy and Infectious Diseases provided a salient example of this of immense biomedical significance. Ronald’s team have been using a combination of intravital multiphoton microscopy and novel multiplex immunohistochemical methods – the latter are called Histo-cytometry and Ce3D – to visualise the immune system, and the findings are surprising. As a PhD student, I puzzled over the relationship between the macrophage and the neutrophil – two main players of the immune system who are often observed together in biopsies of damaged tissue – and conventional wisdom when I was a PhD student was that these distinct cell types work together to orchestrate a local immune response. However, biopsies are just a snapshot in time of a disease process and are notoriously difficult to interpret. Ronald used his live imaging approach to showcase damage-dependent neutrophil swarm behaviour in vivo. Neutrophils swarm to a site of injury, and this neutrophil swarm creates collateral damage to the collagen extracellular matrix. Loss of collagen has a knock-on effect for remodelling damaged tissue, and may explain the perpetuation of inflammatory responses in injured tissue. Ronald additionally showed that ‘cloaking’ by macrophages around the site of injury prevents neutrophils from swarming to this region, and therefore it appears likely that the macrophage serves to dampen the local immune response rather than support neutrophil infiltration, and therefore may help to limit the damage to extracellular matrix. This finding has important ramifications for our knowledge of the regulation of the inflammatory response, and may even inform decisions that are made in the clinic. Ronald’s multiplex imaging approach can capture up to 27 different colours in a tissue sample – the example shown at the symposium was a mesenteric lymph node – and this will be an important method for capturing many of the additional players of the immune system and more accurately defining their role in the context of health and disease.
Learning from Nature – Reflectins in Cephalopods
Reflectins proteins are responsible for the iridescence and light scattering properties of cephalopods, the class of molluscs that includes squid, octopus and nautilus, and represent one means by which these marine animals can change colour and appear transparent. Gerald Pao of the Salk Institute has been exploring what appends when Reflectin proteins are targeted to organelles of mammalian cells, such as irregular ER, where they increased scattering in transfected cells. As Gerald explained, “it is possible to alter the optical refractive index of cellular compartments in vivo in a genetically encodable manner.” Gerald further explained the unusual nature of Reflectin proteins, which are predicted to be an amorphous class of protein that do not fold at all. Using EM, Gerald showed that that Reflectin nanoparticles can exist in two states, and that they are either space filling or particles. Although there is much still to learn about these enigmatic proteins, these studies highlight the tantalizing possibility of changing the optical properties of mammalian tissues, and perhaps even rendering them semi-transparent. If mastered, this would have a huge impact on our ability to observe molecular processes in living organisms.
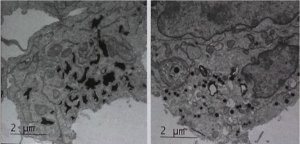
Electron micrographs of Reflectin A2, derived from the Bigfin Reef Squid Sepioteuthis lessoniana, transfected into human embryonic kidney 293T cells. Reflectin nanoparticles can exist in two states, and that they are either space filling (left) or particles (right). Image reproduced with kind permission of Gerald Pao.
Compressed sensing – a solution for super-resolution imaging across time?
There was a very interesting talk by Ola Sabet of the Lucas Pelkmans lab in the University of Zurich that dealt with the concept of sparsity, which is the lack of complexity of an image. If you consider white noise, where every pixel is different, this represents a non-sparse set. In contrast, biological images tend to be sparse. Ola presented the theory of compressed sensing, where the only assumption is that the signal is sparse, and showed that by modulating light emitted from point sources, it is possible to use compressed sensing sparsity-incoherence requirements to generate super-resolution images of living cells. We’ve previously published on this solution being utilised for GWAS data, so it was interesting to see it adapted in another context. As Ola explained, “using a digital grid with a pixel size smaller than the original projected camera pixel size, we can increase the total number of pixels and thereby the spatial resolution in the output image”. This technique can deliver super-resolution images of approximately 50 nanometres resolution in the lateral dimension. Importantly, this imaging technique uses conventional fluorophores, and therefore may have the added value that it allows researchers to explore the temporal domain at super-resolution. Photobleaching is a known issue in super-resolution imaging, and has hampered temporal studies. However, this novel method developed by the Pelkmans lab – known as Brownian Excitation Absorption Microscopy (BEAM) – may represent one method of crossing into what Ola refers to as “the temporal barrier”.
Don’t Stop (Seeing) is Believing
You can read more on the EMBL Seeing is Believing symposium on their blog, which features write ups of all the talks. This was the second time the symposium has been held at EMBL, the previous being in 2017. If this becomes a biennial event we will certainly like to attend again, and hope that like the Journey song the Seeing is Believing symposium goes on and on, and on, and on.
